
The amount of methyl chloride in the stratosphere is estimated to be 2 x 10 6 tonnes per year, representing 20-25% of the total amount of chlorine that is emitted to the stratosphere annually. The half-life of this substance in terms of volatilization in the river, lagoon and lake is 2.1 h, 25 h and 18 days, respectively. On the other hand, when the methyl chloride emitted is released to water, it will be rapidly lost by volatilization. After being released into the air, the atmospheric lifetime of this substance is about 10 months with multiple natural sinks, such as ocean, transport to the stratosphere, soil, etc. Most of the methyl chloride present in the environment ends up being released to the atmosphere.
#Ch3cl polarity free#
Abundances are given as pollution free monthly mean mole fractions in parts-per-trillion. Dispersion in the environment ĬH 3Cl measured by the Advanced Global Atmospheric Gases Experiment ( AGAGE) in the lower atmosphere ( troposphere) at stations around the world. This chlorination method also cogenerates hydrogen chloride, which poses a disposal problem.

For this reason, methane chlorination is usually only practiced when these other products are also desired. This method, however, also produces more highly chlorinated compounds such as dichloromethane, chloroform, and carbon tetrachloride. Ĭhloromethane is produced commercially by treating methanol with hydrochloric acid or hydrogen chloride, according to the chemical equation: CH 3OH + HCl → CH 3Cl + H 2OĪ smaller amount of chloromethane is produced by treating a mixture of methane with chlorine at elevated temperatures. This method is the forerunner for that used today but uses hydrogen chloride instead of sulfuric acid and sodium chloride. Production Ĭhloromethane was first synthesized by the French chemists Jean-Baptiste Dumas and Eugene Peligot in 1835 by boiling a mixture of methanol, sulfuric acid, and sodium chloride. The detections reveal that chloromethane can be formed in star-forming regions before planets or life is formed.Ĭhloromethane has been detected in space. It was also detected in the comet 67P/Churyumov–Gerasimenko (67P/C-G) using the Rosetta Orbiter Spectrometer for Ion and Neutral Analysis (ROSINA) instrument on the Rosetta spacecraft. Interstellar detections Ĭhloromethane has been detected in the low-mass Class 0 protostellar binary, IRAS 16293 –2422, using the Atacama Large Millimeter Array (ALMA).

When contaminated by chloride, this waste burns, releasing methyl chloride in the atmosphere. In the sugarcane industry, the organic waste is usually burned in the power cogeneration process. Sugarcane and the emission of methyl chloride coli, and seems to be present in other organisms such as white rot fungi ( Phellinus pomaceus), red algae ( Endocladia muricata), and the ice plant ( Mesembryanthemum crystallinum), each of which is a known CH 3Cl producer. This protein has been purified and expressed in E. The salt marsh plant Batis maritima contains the enzyme methyl chloride transferase that catalyzes the synthesis of CH 3Cl from S-adenosine-L-methionine and chloride. An extensive study of 30 species of polar macroalgae revealed the release of significant amounts of CH 3Cl in only Gigartina skottsbergii and Gymnogongrus antarcticus. Laboratory cultures of marine phytoplankton ( Phaeodactylum tricornutum, Phaeocystis sp., Thalassiosira weissflogii, Chaetoceros calcitrans, Isochrysis sp., Porphyridium sp., Synechococcus sp., Tetraselmis sp., Prorocentrum sp., and Emiliana huxleyi) produce CH 3Cl, but in relatively insignificant amounts.
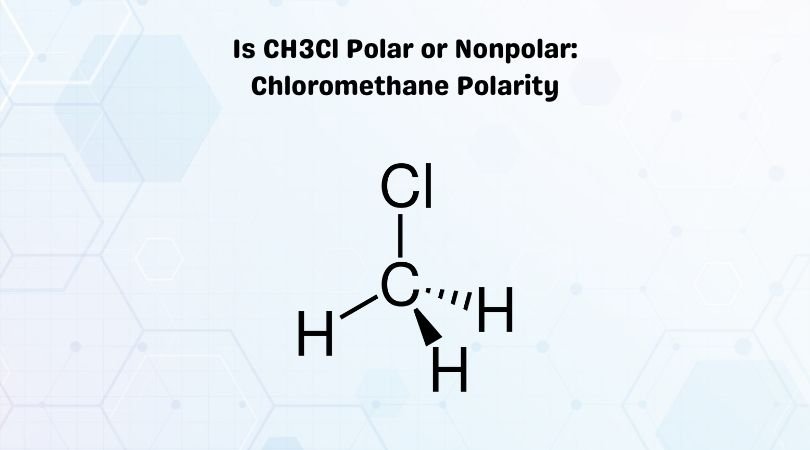
Methyl chloride is a crucial reagent in industrial chemistry, although it is rarely present in consumer products, and was formerly utilized as a refrigerant.Ĭhloromethane is an abundant organohalogen, anthropogenic or natural, in the atmosphere. One of the haloalkanes, it is a colorless, sweet-smelling, flammable gas. Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula CH 3Cl.


 0 kommentar(er)
0 kommentar(er)
